9 Leica Lightning Enhanced Resolution
In the Resolution tutorial, you learned that our best widefield (“normal”) microscopes and objectives have a resolution limit of ~0.2 μm (200 nm). This means that that, even under the best conditions, you cannot resolve details or measure distances smaller than 200 nm. You may be able to see objects smaller than 200 nm, but you will not be able to determine if the object you are seeing is actually one object or many objects very close together and you will not be able to determine its actual size. These are major limitations as many biological objects are smaller than this limit (e.g. proteins, most viruses, ribosomes) or near to it (e.g. most bacteria, synapses). The Leica Stellaris 8 confocal with Lightning is able to resolve details smaller than this limit, theoretically down to ~120 nm.
The Physical Basis of the Optical Resolution Limit
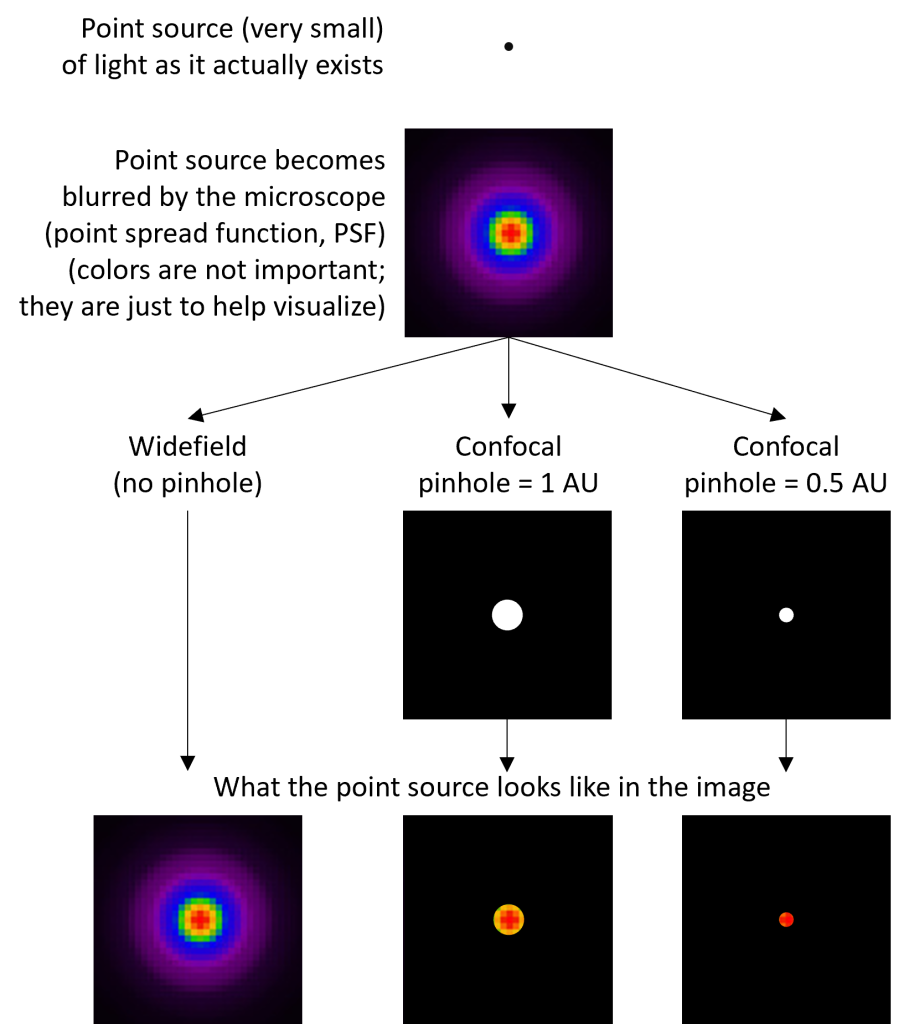
As light travels through a microscope, it becomes distorted. Consider a very small point of light (a “point source”) that is being imaged by a widefield microscope (Fig. 1). The image of the point no longer looks like the actual point, but is distorted into a much larger spot, surrounded by concentric rings of light. The point is also distorted in the axial/z-direction, although that is not illustrated in the figure. This characteristic “blur” is called a point spread function (PSF). The practical outcome of this is that the image does not accurately represent the true dimensions of the point object. The blur also limits how close two objects can be and still be resolved: the greater the blur, the greater the distance the objects must be to be resolved. This is the basis of the optical resolution limit. The shape of the PSF depends on certain properties of your sample (e.g. the wavelengths of light emitted by your fluorescent dye) and microscope (e.g. the objective numerical aperture). It cannot be eliminated, but it can be reduced.
Confocal Microscopy Improves Upon Widefield Resolution
Although both widefield and confocal microscopes are subject to the same limitation described in the preceding paragraph, confocal microscopes can achieve a slightly better resolution because of the action of the confocal pinhole. In addition to excluding out-of-focus light, the pinhole excludes light from neighboring objects (objects not directly on center with the pinhole) and the outer edges of the PSF (Fig. 1). In other words, the pinhole helps separate neighboring objects and limits the size of the PSF (blur).
One could theoretically improve the resolution of a confocal microscope by making the pinhole smaller. However, because only light that passes through the pinhole is detected, shrinking the pinhole also blocks more light, producing a dimmer image and making it difficult to identify an object’s signal above the background noise (a low signal-to-noise ratio, SNR). There is therefore a trade-off between improving resolution and SNR. In practice, confocal pinholes are generally set to 1 Airy-unit (AU), which provides a small increase in resolution and a large improvement in optical sectioning while still maintaining a sufficient SNR.
Deconvolution Improves Upon Widefield Resolution
Deconvolution is a computational process that “reconstructs” images by reversing the blurring effect of the PSF. To understand the process, it’s helpful to consider an analogy. Imagine a detailed drawing made by a sharp pencil or fine-tip pen (Fig. 2). Now imagine that you trace over the object using a fat crayon. The original drawing is convolved (meaning combined or mixed) with the thick tip of the crayon, producing a much less detailed drawing. Now imagine that you know the exact thickness of the crayon tip. With this knowledge you might be able to reconstruct the original drawing by replacing every line that is the thickness of the crayon tip with a thinner line. This process is called deconvolution because you are unmixing the original drawing and the thick crayon. A microscope convolves an object with the PSF (analogous to the fat crayon) to produce a less-detailed image. However, if we know the shape of the PSF, we can computationally reconstruct the original object—i.e. deconvolve the object and the PSF.
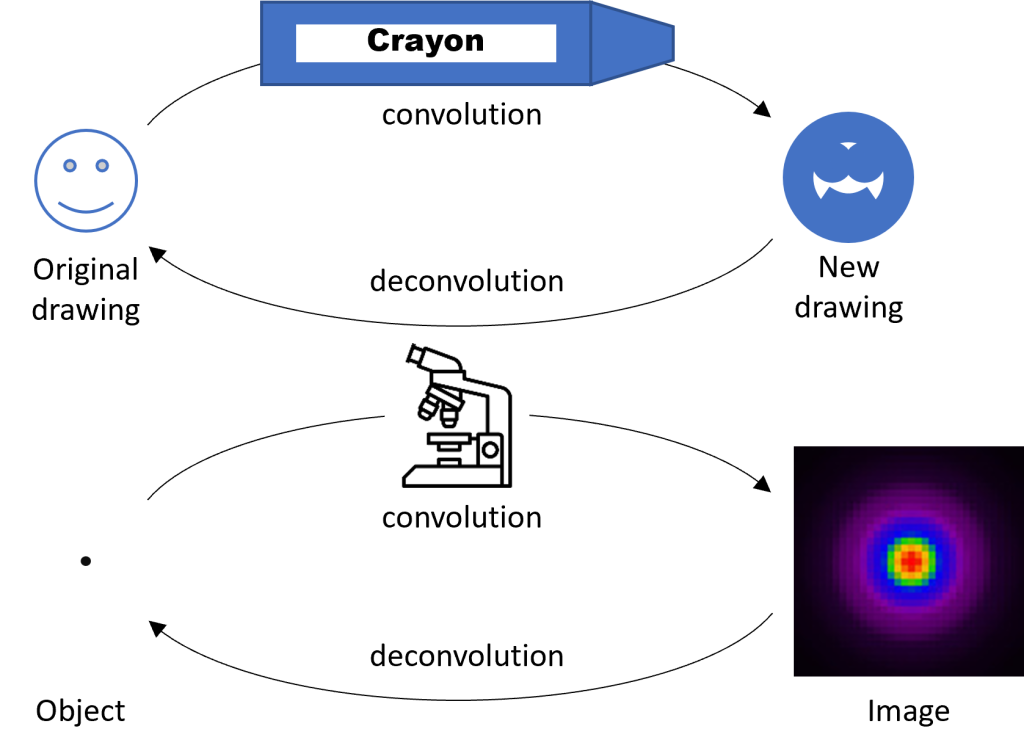
Deconvolution works best when you have a high-SNR image (high signal; low noise) and a very well characterized model of your PSF. Remember that the PSF shape changes based on multiple aspects of your sample and microscope. Although deconvolution can never completely correct for the distorting effects of the PSF, you can reduce blurring and improve the overall resolution of the image similar to the level achieved by confocal microscopy.
Lightning Uses a Small Pinhole and Deconvolution
Is it possible to deconvolve a confocal image, combining the two resolution-enhancing processes and producing an even better resolution image? In theory, yes, but in practice, the SNR of confocal images is typically too low to allow for accurate deconvolution. The Stellaris confocal overcomes this limitation by employing high-sensitivity light detectors that capture more light with a lower level of background noise. These detectors allow the pinhole to be closed-down to as little as 0.5 AU while still producing images with a sufficiently high SNR to allow deconvolution. Using both processes, the resolution limit of the Stellaris can be decreased to approximately half the theoretical resolution limit for a widefield microscope (~120 nm using low-wavelength light and a 1.4 NA objective), i.e. a 2x improvement in resolution.
Practical Aspects of Using Lightning
Using Lightning might not be necessary for your experiment
The Stellaris confocal microscope can operate in “normal” mode (pinhole = 1 AU, no deconvolution) or in Lightning mode (control over pinhole, with deconvolution). Even though Lightning mode will produce higher-resolution images, there are several reasons why you might not want to use it. For example, Lightning usually decreases imaging speed and produces larger file sizes. In addition, depending on the size of the objects you are imaging, you might not need higher resolution images—standard confocal images may be sufficient. Finally, note that Lightning is incompatible with the Falcon quantitative fluorescence lifetime imaging (FLIM) but is compatible with TauSense qualitative FLIM.
Lightning controls more than just the pinhole and deconvolution
Closing-down the confocal pinhole decreases the resolution limit (better resolution), decreases the optical section thickness (less out-of-focus light), and decreases light collection (dimmer image). Therefore, when you change the pinhole size, you also need to adjust several other imaging parameters to compensate for these “side effects.”
- Changes to the optical resolution limit require you to adjust your digital resolution. Remember from the Resolution tutorial that your pixel size should be less than or equal to one-third the smallest distance you want to resolve. Therefore, if closing-down the pinhole allows you to resolve smaller distance, you need to reduce your pixel size accordingly.
- Changes to the optical section thickness require you to adjust your z-step size for z-stacks. The reason for this is analogous to the previous point. (Note that you must acquire a z-stack—even a small one—for deconvolution to work properly.)
- Changes to light collection require you to adjust your other acquisition parameters to maintain a sufficient SNR. There are many parameters that can be adjusted including the laser power, detector gain, pixel dwell time, and line/frame averaging.
Because of the complexity of making these adjustments, Lightning includes a simple option that allows you to control everything with just one slider. It adjusts the pinhole as well as all the other parameters to compensate.
Sometimes it makes sense to use a larger pinhole with Lightning
One interesting feature of Lightning is that it allows you to increase the pinhole size. This actually makes your optical resolution worse, so why would you want to do it? The secret is that most of Lightning’s improvement in resolution comes from the deconvolution procedure and not from the smaller pinhole. A larger pinhole allows more light to be collected and produces a brighter image. This allows you to acquire a brighter image more quickly and gently (less photobleaching and photodamage). Because the deconvolution step will still improve your resolution, you may still achieve an overall higher resolution than a normal confocal image, even with using a larger pinhole.
High-quality deconvolution depends on many parameters
As mentioned earlier, deconvolution quality is highly dependent on having an accurate model of the PSF. Many aspects of the microscope and your sample affect the shape of the PSF and you must therefore pay close attention to the following parameters:
- You need to know the refractive index of your specimen as accurately as possible. For fixed samples, this depends primarily on your mounting media. For thin, live samples, the refractive index is usually close to that of water.
- You must have your sample mounted with a #1.5 glass coverslip (thickness = 0.17 µm).
- You must use the correct Leica immersion medium for your objective.
- If your objective has a correction collar, it must be set correctly.
Do a “reality check” of your Lightning images
Lightning will show you your image before and after deconvolution. Because deconvolution can sometimes introduce artifacts (especially if parameters are incorrect), you should always compare your before and after images and also ask whether your final deconvolved images make sense biologically. If your final images show a result that seems too wild to be true, it probably is.
If you use Lightning, you need to be extra thorough in reporting your methods
Because deconvolution depends on many parameters and can sometimes introduce artifacts, you must report exactly how your images were acquired and processed. Luckily, all the critical instrument parameters that you need to report are included in the Leica .lif file. In addition to reporting the standard set of microscope parameters (see the Data Management chapter), you must at a minimum report the pinhole size, refractive index value, and deconvolution strategy (adaptive, global). In addition, it is good practice to make the raw and processed images publicly available so that others can compare them and access the images’ full metadata.

